Objetivos: Estudiar las alteraciones nutricionales en adolescentes con anorexia nerviosa y establecer si ciertas deficiencias persisten tras la recuperación de peso a corto plazo.
Método: Sesenta y una paciente de entre 10 y 19 años ingresadas en una Unidad de Referencia para Trastornos de la conducta Alimentaria, que cumplían criterios diagnósticos DSM-IV para anorexia nerviosa fueron evaluadas en el momento del ingreso y al alta hospitalaria tras la recuperación de peso. Se determinaron una amplia serie de parámetros bioquímicos, nutricionales y hormonales.
Resultados: En el momento del ingreso no se encontraron deficiencias en las proteínas o lípidos, pero un número elevado de pacientes presentaron alteraciones hormonales y déficit de ácido fólico eritrocitario y zinc. Tras la realimentación, en el momento del alta, las alteraciones hormonales mejoraron de forma significativa (p<0, 001). Sin embargo, disminuyeron los hematíes y la hemoglobina (p<0, 001) y el ácido fólico (p<0, 05). El ácido fólico eritrocitario y el zinc aumentaron pero no alcanzaron niveles normales.
Conclusiones: En una amplia proporción de adolescentes con anorexia nerviosa estaría recomendada la administración de suplementos de ácido fólico y zinc durante la recuperación nutricional. La administración de suplementos proteínicos y hormonales no parecen ser necesarios si se logra la recuperación de peso.
Persistencia de déficits nutricionales tras la recuperación de peso a corto plazo en adolescentes con anorexia nerviosa.
(Persistence of nutritional deficiencies after short-term weight recovery in adolescents with anorexia nervosa. )
J. Castro*; R. Deulofeu**; A. Gila*; J. Puig*; J. Toro*.
*Servicio de psiquiatría y psicología Infantil y Juvenil.
**Servicio de Bioquímica Clínica. hospital Clínico Universitario de Barcelona.
PALABRAS CLAVE: Déficits nutricionales, anorexia nerviosa, Adolescentes.
(KEYWORDS: Nutritional deficiencies, anorexia nervosa, Adolescents. )
Resumen
Objetivos: Estudiar las alteraciones nutricionales en adolescentes con anorexia nerviosa y establecer si ciertas deficiencias persisten tras la recuperación de peso a corto plazo.
Método: Sesenta y una paciente de entre 10 y 19 años ingresadas en una Unidad de Referencia para Trastornos de la conducta Alimentaria, que cumplían criterios diagnósticos DSM-IV para anorexia nerviosa fueron evaluadas en el momento del ingreso y al alta hospitalaria tras la recuperación de peso. Se determinaron una amplia serie de parámetros bioquímicos, nutricionales y hormonales.
Resultados: En el momento del ingreso no se encontraron deficiencias en las proteínas o lípidos, pero un número elevado de pacientes presentaron alteraciones hormonales y déficit de ácido fólico eritrocitario y zinc. Tras la realimentación, en el momento del alta, las alteraciones hormonales mejoraron de forma significativa (p<0, 001). Sin embargo, disminuyeron los hematíes y la hemoglobina (p<0, 001) y el ácido fólico (p<0, 05). El ácido fólico eritrocitario y el zinc aumentaron pero no alcanzaron niveles normales.
Conclusiones: En una amplia proporción de adolescentes con anorexia nerviosa estaría recomendada la administración de suplementos de ácido fólico y zinc durante la recuperación nutricional. La administración de suplementos proteínicos y hormonales no parecen ser necesarios si se logra la recuperación de peso.
Abstract
Objectives: To study nutritional abnormalities in adolescent anorexia nervosa and to establish whether certain abnormalities persist after short-term refeeding.
Method: Sixty-one patients aged 10 to 19 admitted to a Reference Unit for Eating Disorders between 1999 and 2000, with DSM-IV diagnosis of anorexia nervosa were evaluated at admission and at discharge. A range of biochemical, nutritional and hormonal parameters were determined.
Results: At admission no protein or lipid deficiencies were found, although many patients presented hormonal abnormalities, and red blood cell folate and zinc deficiencies. . Hormonal abnormalities reverted significantly (p< . 000) after renutrition. There were decreases in erythrocytes and hemoglobin (p< . 000) and folic acid (p< . 05). Red blood cell folate and zinc increased but did not reach normal levels.
Conclusions: In a large proportion of adolescent anorexic patients supplementation of folic acid and zinc is recommended although protein or hormonal replacement does not seem to be necessary.
Malnutrition in anorexia nervosa can be severe but assessment of nutritional status yielded inconsistent and sometimes contradictory results. This may be due to heterogeneity in age and duration of disorder, or to small sample size in some studies (1-4). Among the features most commonly described are anemia and leucopenia (5, 6) which appear to be related to bone marrow changes, though the mechanisms involved are not well known at present (7).
However, these hematological abnormalities are not seen in all studies (8). Protein levels are also normal in many cases (9), albeit in the low-normal range.
Impairment of micronutrient status, especially iron and zinc, is also frequent (10-13) but reports of highly abnormal levels of vitamins (except for folic acid) are surprisingly rare (8, 9, 14). A thiamin deficit was found in 19% of a sample of adult anorexics (15), and it is common to find higher carotene concentrations (16, 17).
Nevertheless, many of these studies were carried out with adult anorexics of long duration and it is not clear whether young adolescents, in whom the evolution time of the disorder is shorter, present marked biochemical and nutritional deficiencies.
Endocrine abnormalities are also common in malnourished anorexia nervosa patients, especially low levels of thyroid hormones (16, 18) and insulin-like growth factor-I (IGF-I) and its binding protein 3 (IGBP-3) (19-23). Among the most frequent alterations in anorexia nervosa are abnormalities of the hypothalamic-pituitary-gonadal axis, and impairment of gonadotropin secretion pattern, which is similar to that of the prepubertal stage (4).
The great majority of studies are cross-sectional, making it impossible to establish whether vitamins or other micronutrients normalize after weight recovery or whether special nutritional supplements may be necessary (24). In this respect, Rock and Vasantharajan (3) found a normalization of vitamin abnormalities in 13 patients with renutrition in about 2-6 weeks. Caregaro et al. (22) found a prompt recovery of IGF-I in 20 adult anorexic patients after short term weight increase. In a study of 33 eating disordered patients, McClain et al. (13) found that zinc supplementation during hospitalization increased their plasma zinc level significantly in comparison with patients without supplement. Nevertheless, no clear guidelines have yet been published for vitamins or trace metals or other micronutrient supplementation in young patients with anorexia nervosa (25, 26).
The objective of the present study was to determine whether adolescent patients with short duration anorexia nervosa have abnormal biochemical, nutritional and hormonal parameters and whether these alterations may be reversed after short term weight recovery. The results of the present study will allow us to establish the rationale for using vitamin or other micronutrient supplementation along with normal refeeding during hospitalization.
Method
Subjects and procedures
The group of patients comprised 61 children and adolescents aged 10 to 19 years who fulfilled the DSM-IV diagnostic criteria (27) for anorexia nervosa. All patients were consecutively admitted to the Reference Unit for Eating Disorders of the Child and Adolescent Psychiatry and Psychology Department of the hospital Clínic Universitari of Barcelona as inpatients between March 1999 and June 2000. Clinical characteristics were recorded. Laboratory data were collected at admission and at discharge as part of the normal follow-up of anorexia nervosa in our unit. Parents and patients were told why biochemical and hormonal parameters were being measured. Study procedures were approved by the Ethics Committee of the Institution. Patients with concomitant disease besides anorexia nervosa or those receiving hormonal therapy or any vitamin complex at admission were excluded from the analysis.
During hospitalization all patients received a complete diet of about 1250 calories per day during the first days which increased progressively to 2500 calories per day, but they did not receive vitamin or mineral supplements or any hormonal replacement therapy. Treatment during admission is based on a multidisciplinary approach combining biological management, nutritional rehabilitation, a behavioral program aimed to improve eating patterns and weight, individual and group cognitive treatment, and individual and group parent counseling.
Laboratory test
The biochemical and hematological tests carried out at admission and at discharge included all standard biochemical data, nutritional parameters, total proteins, prealbumin, ions (potassium, sodium, magnesium, phosphorus, calcium, iron and zinc), vitamins (Vitamins B1, B6, B12, C, E, alfa and beta carotenes), folic acid, and red blood cell folate, a complete hemogram including red blood cell count, hemoglobin, leucocytes and platelet counts and endocrine parameters such as cortisol, triiodothyronine (T3), thyroxin (T4), thyrotropin (TSH), IGF-I, IGFBP-3 and growth hormone (GH). All samples were taken in the morning. Gonadotropin levels were not considered for the present study since they have been extensively studied elsewhere, and because 14 (24. 1%) of the female patients were still prepubertal.
Standard biochemical and hematological parameters were measured in an Olympus AU450 analyzer, (Cormedica, Barcelona, Spain) using Olympus own reagents. Vitamin B12, and folic acid was measured by immunoassay using an automated Immuno 1 system from Bayer (Quimica Farmaceutica Bayer, Barcelona, Spain). Vitamins B1 and B6 were measured by HPLC using Chromsystems reagents (Chromsystems, Munich, Germany). Vitamin C, was measured by HPLC by fluorimetric detection. Vitamin E and carotenes were also measured by HPLC using UV or visible detection respectively. Hematological parameters were measured in an Advia hematology analyzer (Bayer, Quimica Farmaceutica Bayer, Barcelona, Spain). Cortisol, HGH, IGF and IGFBP3 were measured by commercially available radioimmunoassay as routinely measured at our institution.
Statistical analysis
Differences between mean initial values and follow-up measurements were analyzed using the Student’s t test for paired samples. The McNemar test was used to compare the incidence of abnormal parameters in the first and second determinations. The level of statistical significance was p< . 05. Statistical analysis was performed using the SPSS package (28).
Results
General Characteristics
Mean age of patients was 14. 9 years (SD=2. 1), 58 (95. 1%) patients were females and 3 (4. 9%) were males. Mean body mass index at admission was 15. 3 (SD=1. 3), and the mean percentage of weight loss was 23. 1% (SD=7. 6). Mean period between the onset of the disorder (i. e. , since the first weight loss) and admission was 15. 3 months (SD=10. 3). Fourteen (24. 1%) female patients were prepubertal. The mean period of amenorrhea in patients with secondary amenorrhea was 9. 1 months (SD=7. 7). Fifty-two (85. 2%) were restrictive type and 9 (14. 8%) were purgative. Table 1 shows patients data for anthropometric and demographic characteristics, , including weight gain and stay time. Treatment efficiency was shown by a signifcant increase in BMI after stay.
Tabla 1
Laboratory findings
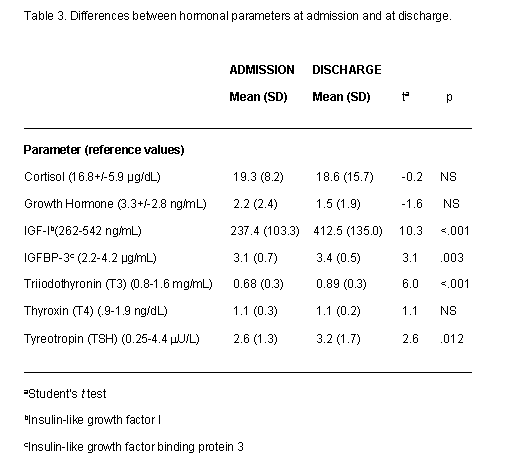
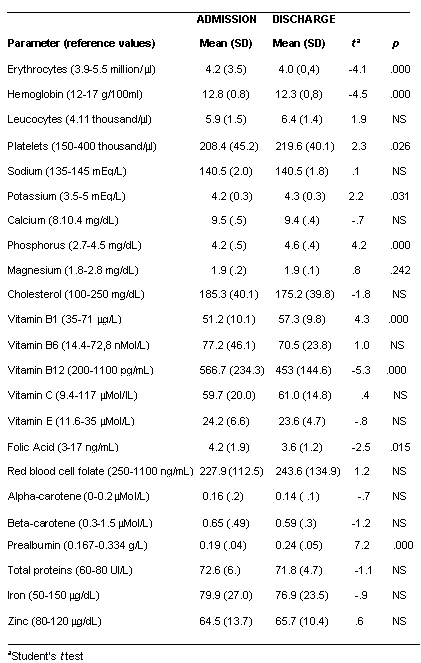
Results for the biochemical, nutritional and hormonal parameters are shown in tables 2 and 3 as means ±SD. The tables also present differences between admission and discharge
Tabla 2
Tabla 3
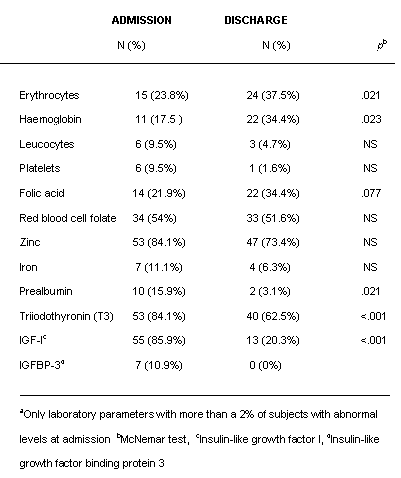
In general, hematological and basic biochemical parameters fell within normal ranges although many of them were low to normal. At entry the only nutritional parameters with a mean level below reference values were red blood cell folate and zinc. Of the hormonal parameters, IGF-1 and T3 also had mean scores below reference values. Parameters in the low-normal range were total red blood cells, hemoglobin, iron, serum folic acid and prealbumin. All other micronutrients were within normal ranges apart from folic acid and zinc, as mentioned above.
Surprisingly, after short-term weight recovery, mean values of erythrocyte count, hemoglobin and folic acid decreased significantly. In contrast, the concentrations of prealbumin increased and normalized; red blood cell folate, and zinc also increased, but they did not reach normal values and the differences pre- and post- weight recovery were not statistically significant. Among hormonal determinations, IGF-I, IGFBP-3 and T3 normalized after weight recovery.
Prevalence of abnormal values
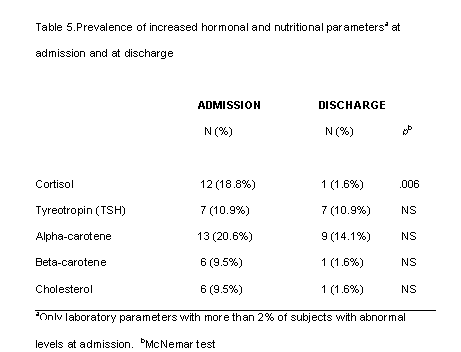
Although the majority of mean values were normal in this group of patients, some presented abnormal nutritional and hormonal parameters (tables 4 and 5). For this analysis we only considered laboratory parameters for which more than 2% of subjects presented abnormal levels at admission. The rest of the parameters listed in tables 2 and 3 were normal in more than 98% of subjects at both time points. Table 4 shows the percentages of subjects with low nutritional and hormonal determinations at admission and at discharge. More than 80% of subjects had low levels of T3, IGF-I and zinc at admission. More than 50% had low levels of red blood cell folate and more than 20% low levels of erythrocytes and plasma folic acid. The effect of refeeding was especially clear on T3 and IGF-1 and the percentages of patients with normal values for these parameters increased significantly. At discharge abnormal levels of zinc and red blood cell folate remained in a substantial proportion of subjects and, surprisingly, the percentage of patients with suboptimal levels of erythrocytes, hemoglobin and plasma folic acid increased.
Tabla 4
Table 5 shows the percentages of subjects with high levels of certain parameters at admission and at discharge. In this case high levels of cortisol were found in 19% of subjects and high levels of alpha-carotenes in 20% but at discharge the number of patients with these levels had decreased.
Tabla 5
Discussion
An increased prevalence of nutritional deficiencies can be considered the main issue of this study although no basic biochemical abnormalities could be observed. Probably these findings are due to the fact that many parameters are under strict homeostatic control and are unlikely to be altered without longer-term deficiencies or till organ damage exist. In our study patients were very young and the duration of starvation or nutritional restrictions were relatively short. Low levels of red blood cell counts, leucocytes and platelets have often been reported in studies of anorexia nervosa patients (6) though not in all (8). In our sample, at admission there were more patients with low levels of erythrocytes and hemoglobin than with low levels of leucocytes and platelets. Furthermore, after short-term weight recovery, the percentage of patients with low leucocytes and platelets fell, while the percentage of patients with low erythrocytes and hemoglobin rose. The plasma level of folic acid was in the low-normal range and red blood cell folate was clearly below normal; these are factors that may have an important role in the development of anemia. After refeeding, the percentage of patients with low red blood cell folate was still very high and the percentage of patients with abnormal plasma folic acid increased, suggesting the need for extra folic acid supplementation during renutrition. Other authors have pointed out the possibility of a folate deficiency in adolescents with anorexia nervosa (29) and folate deficiency has also been related to depressive symptoms in several psychiatric disorders, including anorexia nervosa (30). It seems that a supplementation of folic acid may be appropriate for management of this group of patients. Although few subjects had low serum levels of iron (11%), and the mean level was normal, though in the low range , iron supplementation may be indicated in these patients to avoid deficit appearance during folic acid supplementation.
With the exception of folic acid, no absolute vitamin deficiency was seen in this study, again probably due to the fact that the subjects were adolescents with short duration of the disorder. Other authors have not found vitamin abnormalities in anorexia nervosa patients (8), and Rock and Vasantharajan (3) found suboptimal vitamin status in 4 out of 13 patients, which normalized after short-term refeeding. Winston et al. (15) found a thiamin deficiency in about 19% of 37 adult anorexic patients, but this was not the case in the present study. Serum concentrations of alpha-carotene were high in 20% of subjects at admission. Other studies have found that alpha-carotene levels were significantly higher in patients with a greater degree of eating pathology (31). The percentage of subjects with high beta-carotene was lower than in other studies (17) although in our country mean serum values of carotenes are lower than in other Caucasian populations (32).
Total protein levels were normal in all patients at admission and at discharge but prealbumin, the most sensitive marker of protein depletion, was low in 16% of patients at admission a fact which is never found in healthy adolescents . Only 3% of patients had abnormal prealbumin at discharge, demonstrating the efficacy of the refeeding strategy for protein status . Rigaud et al. (9) concluded that protein markers are not sensitive to malnutrition in anorexia nervosa, but this may have been because they considered only total proteins, and albumin does not react so fast in this situation, due to the fact that prealbumin has a half life of 48 hours and albumin of 19 days (33).
Zinc is a very important ion in many cell processes and also in brain homeostasis (34). The finding of such low levels and the lack of recovery after the refeeding period suggests the need to accelerate zinc recovery with supplementation. Other studies have found low levels of zinc in anorexic patients, which did not increase with short-term renutrition if supplementation was not given (13) and it has been suggested that zinc deficiency may be one of the factors that caused altered eating behavior, anxiety and depressive symptoms to persist (11, 12). In other populations such as elite athletes, unusual nutritional habits may also lead to suboptimal zinc intake and mild zinc deficiency (35). It is well known that zinc deficiency may induce anorexia, loss of weight, ageusia, hair loss and mood changes.
The hormones most likely to present abnormalities seem to be T3 and IGF-I, as other studies have pointed out (19, 20, 22, 23)). With malnutrition they are low in the great majority of cases, but with weight recovery levels of T3 and IGF-I reach normal values in the short term. Recovery of IGF-I may be related to bone formation and to the improvement in bone mass (23, 36).
In summary, a great percentage of adolescent patients presented nutritional and hormonal abnormalities, although none had severe vitamin deficiencies. It seems that the most persistent abnormalities after short-term weight recovery in adolescents with anorexia nervosa are low levels of erythrocytes, hemoglobin, folic acid and zinc.
Furthermore folic acid deficit may account for the persistence of anemia. Other abnormalities tend to normalize fast after short-term refeeding, and therefore it does not seem advisable to add protein or vitamin supplementations to the normal diet. The main conclusion is that extra supplements of folic acid, iron and zinc could be beneficial to rapidly compensate deficits that cannot be recovered only with short standard nutrition programs. These supplements may contribute to ameliorating anemia and folate and zinc deficiencies, and to improve certain psychological abnormalities . Further controlled studies of the effects of these supplements on both physical and psychological symptomatology would be of great interest.
References
1. Rock CL, Curran-Celentano J. Nutritional disorder of anorexia nervosa:
a review. International J Eat Disord 1994, 15:187-203.
2. Langan SM, Farrell PM. Vitamin E, vitamin A and essential fatty acids status of patients hospitalized for anorexia nervosa. Am J Clin Nutrition 1985, 41:1054-1060.
3. Rock CL, Vasantharajan S. Vitamin status of eating disorder patients: relationship to clinical indices and effect of treatment. Int J Eat Disord 1995, 18:257-262.
4. Van Binsbergen CJ, Coelingh Bennink HJ, Odink J, Haspels AA, Koppeschaar HP.
A comparative and longitudinal study on endocrine changes related to ovarian
function in patients with anorexia nervosa. J Clin Endocrin Metab 1990, 71:705-711.
5. Brotman AW, Rigotti N, Herzog DB. Medical complications of eating
disorders: Outpatient evaluation and management. Comprehensive Psychiatry 1985,
26:258-272.
6. Lambert M, Hubert C, Depresseux G, Vande Berg B, Thissen JP, Nagant de Deuxchaisnes C et al. Hematological changes in anorexia nervosa are correlated with total body fat mass depletion. Int J Eat Disord 1997, 2:329-334.
7. Geiser F, Murtz P, Lutterbey G, Traber F, Block W, Imbierowicz D et al. Magnetic resonance spectroscopic and relaxometric determinations of bone marrow changes in anorexia nervosa. Psychosom Med 2001, 63:631-637.
8. Van Binsbergen CJ, Odink J, Van den Berg H, Koppeschaar H, Coelingh
Benninck HJ. Nutritional status in anorexia nervosa: clinical chemistry,
vitamins, iron and zinc. Eur J Clin Nutrition 1988, 42:929-937.
9. Rigaud D, Sogni P, Hammel P, Melchior JC, Angel L, Rozen R et al. anorexia nervosa: absence of sensitivity to nutritional protein markers. Study of 23 patients and comparison to a paired group with colonic Crohn’s disease. Annals Med Interne 1989, 140:86-90.
10. Casper RC, Kirschner B, Sandstead HH, Jacob RA, Davis JM. An evaluation of trace metals, vitamins, and taste function in anorexia nervosa. A J Clin Nutrition 1980, 33:1801-1808.
11. Katz RL, Keen CL, Litt IF, Hurley LS, Kellams-Harrison KM, Glader LJ. Zinc deficiency in anorexia nervosa. Journal of Adolescent Health Care 1987, 8, 400-406.
12. Humphries L, Vivian B, Stuart M, McClain CJ. Zinc deficiency and eating disorders. J Clinl Psychiatry 1989, 50:456-459.
13. McClain CJ, Stuart MA, Vivian B, McClain M, Talwalker R, Snelling L et al. Zinc status before and after zinc supplementation of eating disorder patients. J Am College Nutrition 1992, 11:694-700.
14. Philipp E, Pirke KM, Seidl M, Tuschl RJ, Fichter MM, Eckert M et al. Vitamin status in patients with anorexia nervosa and bulimia nervosa. Int J Eat Disord 1988, 8:209-218.
15. Winston AP, Jamieson CP, Madira W, Gatward NM, Palmer RL. Prevalence of thiamin deficiency in anorexia nervosa. Int J Eat Disord 2000, 28:4 51-454.
16. Currant-Celentano J, Erdman JW Jr, Nelson RA, Grater SJ. Alterations in vitamin A and thyroid hormone status in anorexia nervosa and associated disorders. Am J Clin Nutrition 1985, 42:1183-1191.
17. Boland B, Beguin C, Zech F, Desager JP, Lambert M. Serum beta
carotene in anorexia nervosa patients: A case control study. Int J Eat Disord 2001,
30:299-305.
18. Kiyoara K, Tamai H, Takaichi Y, Nakagawa T, Kumagai LF. Decreased thyroidal triiodothyronine secretion in patients with anorexia nervosa: influence of weight recovery. Int J Clin Nutrition 1989, 50:767-762.
19. Counts DR, Gwirstman H, Carlsson LM, Lesem N, Cutler GB Jr .
The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the
insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol
Metab 1992, 75:762-767.
20. Argente J, Caballo N, Barrios V, Muñoz MT, Pozo J, Chowen JA et al. Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in patients with anorexia nervosa: effect of short- and long-term weight recuperation. J Clin Endocrinol Metab 1997, 82:2084-1092.
21. Stoving RK, Flyvbjerg A, Frystyk J, Fisker S, Hangaard J, Hansen-Nord M et al.
Low serum levels of free and total insulin-like growth factor I (IGF-
I) in patients with anorexia nervosa are not associated with increased IGF-binding
protein-3 proteolisis. J Clin Endocrinol Metab 1999, 84:1346-1350.
22. Caregaro L, Favaro A, Santonastaso P, Alberino F, Di Pascoli L, Nardi M et al. Insulin-like growth factor 1 (IGF-1), a nutritional marker in patients with eating disorders. Clin Nutrition 2001, 20:251-257.
23. Heer M, Claudia M, Grzella I, Drummer Ch, Herpertz-Dahlmann B.
Changes in bone turnover in patients with anorexia nervosa during eleven weeks of
inpatient dietary treatment. Clin Chemistry 2002, 48:754-760.
24. Kennedy SH, Shapiro C (1993). Medical management of the hospitalized patient. In: Kaplan AS, Garfinkel PE, editors. Medical Issues and the eating disorders. The interface. New York: Brunner/Mazel Pub. ; 1993. p. 213-238.
25. Steiner H, Look J. anorexia nervosa and bulimia nervosa in children and adolescents: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry 1998, 37:352-359.
26. American Psychiatric Association. Practice guidelines for the treatment of patients with eating disorders. Am J Psychiatry 2000, 157 (Supplement).
27. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV). Washington, DC: American Psychiatric Association; 1994.
28. Norusis MJ. SPSS for windows base system user’s guide, Release 6. 0.
Chicago: SPSS Inc. ; 1993.
29. Moyano D, Vilaseca MA, Artuch R, Valls C, Lambruschini N. Plasma total-homocysteine in anorexia nervosa. Eur J Clin Nutrition 1998, 52:172-175.
30. Abou-Saleh MT, Coppen A. The biology of folate in depression: implications for nutritional hypotheses of the psychoses. J Psychiatric Res 1986, 20:91-101.
31. Rock CL, Gorenflo DW, Drewnowski A, Demitrac MA. Nutritional
characteristics, eating pathology, and hormonal status in young women. Am J Clin
Nutrition 1996, 64:566-571.
32. Olmedilla B, Granado F, Gil-Martinez E, Blanco I, Rojas-Hidalgo E. Reference values for retinol, tocopherol, and main carotenoids in serum of control and insulin dependent diabetic Spanish subjects. Clin Chemistry 1997, 43:1066-1071.
33. Tietz N. Proteins. In: Burtis CA, Ashwood ER, editors. Textbook of
Clinical Chemistry. 2nd edition, Philadelphia: WB Saunders Co. ; 1994. p. 700-704.
34. Fredickson CJ. Neurobiology of zinc and zinc-containing neurons.
Int Review Neurobiology 1989, 31:145-238
35. Micheletti A, Rossi R, Rufini S. Zinc status in athletes: Relation to diet and exercise. Sports Medicine 2001, 31:577-582.
36. Castro J, Lázaro L, Pons F, Halperin I, Toro J. Adolescent anorexia
nervosa: the catch-up effect in bone mineral density after recovery. Journal of the
Am Acad Child Adolesc Psychiatry 2001, 40:1215-1221.
IMPORTANTE: Algunos textos de esta ficha pueden haber sido generados partir de PDf original, puede sufrir variaciones de maquetación/interlineado, y omitir imágenes/tablas.
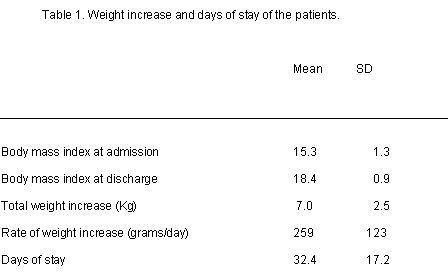
Programa terapéutico digital para adolescentes
Lorena Martin
Fecha Publicación: 01/11/2025
A solas con Francisco Ferre
Adamed Laboratorios
Fecha Publicación: 30/07/2025
Comportamiento de los adolescentes y jóvenes ante la publicidad.
Aurora Suárez Gomez et. al
Fecha Publicación: 19/05/2025
Factores psicológicos implicados en adicciones en población adolescente
Lidia María Sanz Martín et. al
Fecha Publicación: 18/05/2025
La influencia de la comunicación de supermercados españoles en el cerebro de los adolescentes
María Suarez
Fecha Publicación: 18/05/2025
Modelo teórico de formación en salud sexual y reproductiva de adolescentes de secundaria básica, en el vínculo escuela-familia-comunidad
Sandra Ochoa Durán
Fecha Publicación: 18/05/2025